IQ OQ PQ, Process Validation, Equipment Validation
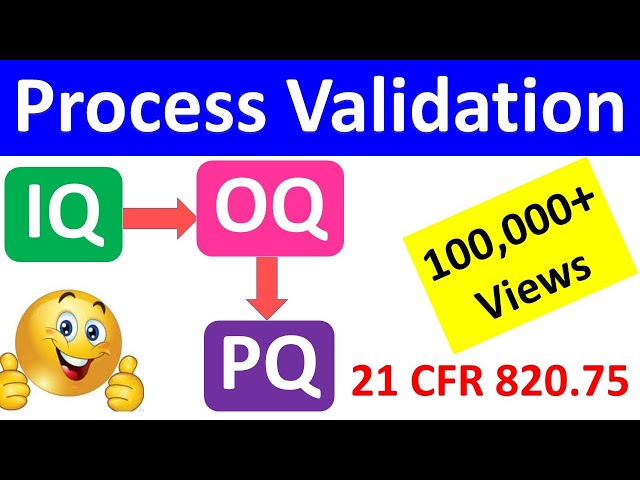
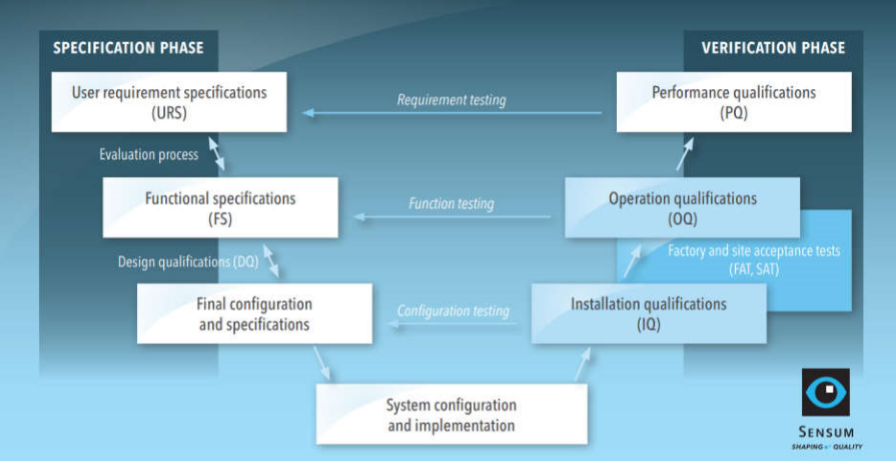
IQ OQ PQ are 3 pillars of Process Validation. IQ stands for Installation Qualification. OQ is Operational Qualification and PQ is Performance Qualification.
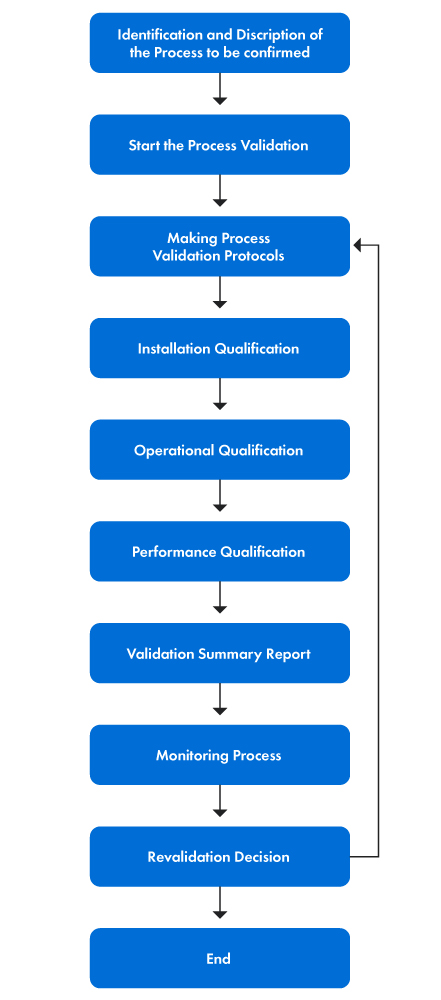
Steps involved in US FDA's Medical devices Validation Protocol Process

What is Qualification Protocol (IQ/OQ/PQ/IFQP), Its Necessity & When re-qualification performed in CSV? – Be Innov@tive

IQ, OQ, PQ: A Quick Guide to Process Validation
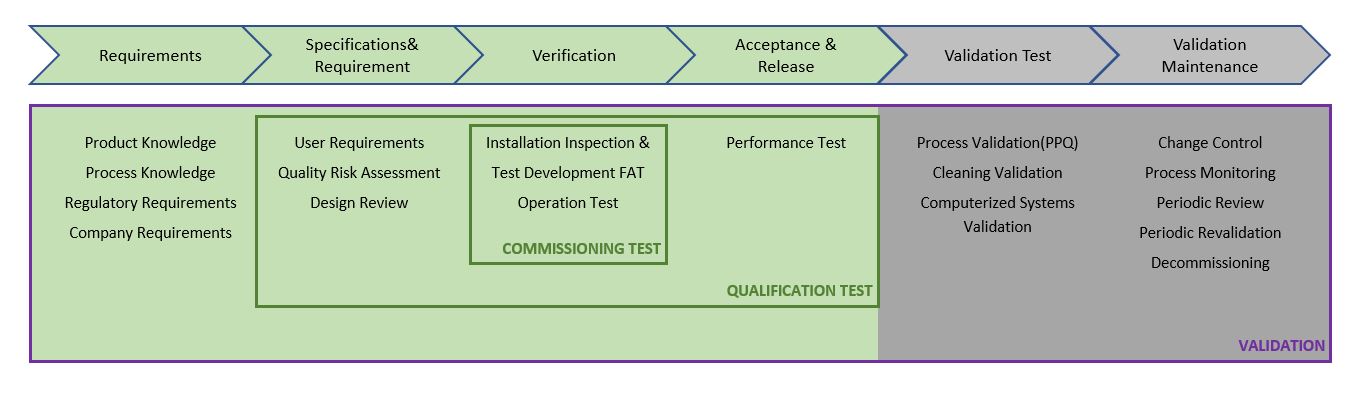
IQ, OQ, and PQ in the Pharmaceutical Industry – No deviation

Equipment Validation : PresentationEZE

Equipment And Process Validation Taking your injection molding business to the next level
ISO 13485 Process Validation Procedure Bundle

IQ OQ PQ, Process Validation, Equipment Validation, Equipment Qualification
Pharmaceutical qualification and validation: tips to get through nightmares

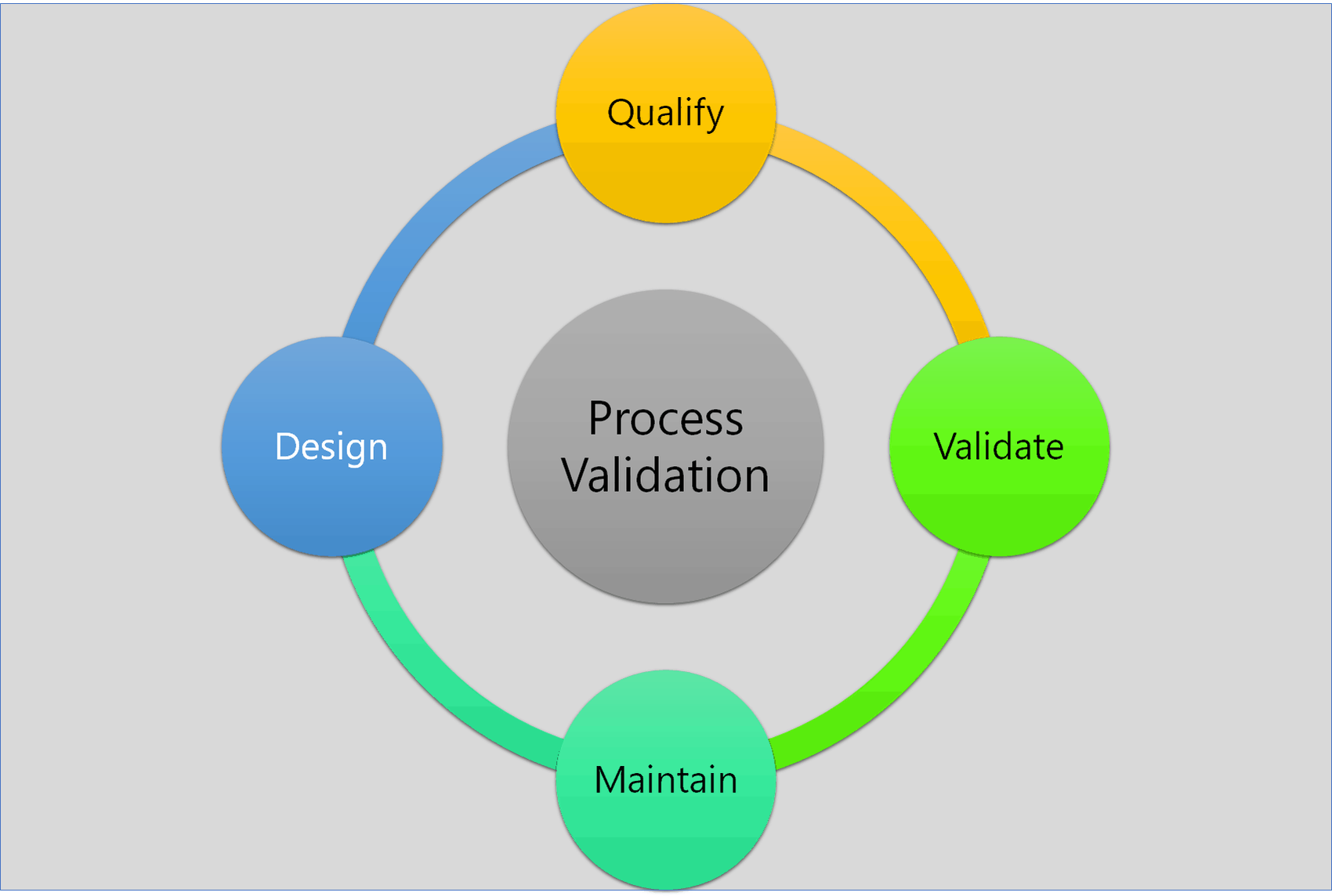
Mastering Process Validation: An In-Depth Guide to IQ/OQ/PQ in Biomanufacturing
Process Validation: The Essential Guide to Ensuring Product Quality and Compliance - Pharma GxP

IQ, OQ, and PQ Validation Services – GK BioScience




:format(webp):quality(70)/https%3A%2F%2Fmedia.topito.com%2Fwp-content%2Fuploads%2F2023%2F02%2FPQVeutDireDeToi.jpg)


