December 27, 2022, Deadline for Mandatory Rx Data Collection Reporting

As group health plan sponsors, employers are responsible for ensuring compliance with the prescription drug data collection (RxDC) reporting requirements

Tri-agencies provide good faith reliance relief and grace period for CMS RxDC reporting obligations

Second Prescription Drug (RxDC) Report for Pharmacy Transparency in Health Plans Is Due by June 1, 2023

Second Prescription Drug (RxDC) Report for Pharmacy Transparency in Health Plans Is Due by June 1, 2023

Federal Register :: Medicare Program; Contract Year 2024 Policy and Technical Changes to the Medicare Advantage Program, Medicare Prescription Drug Benefit Program, Medicare Cost Plan Program, Medicare Parts A, B, C, and
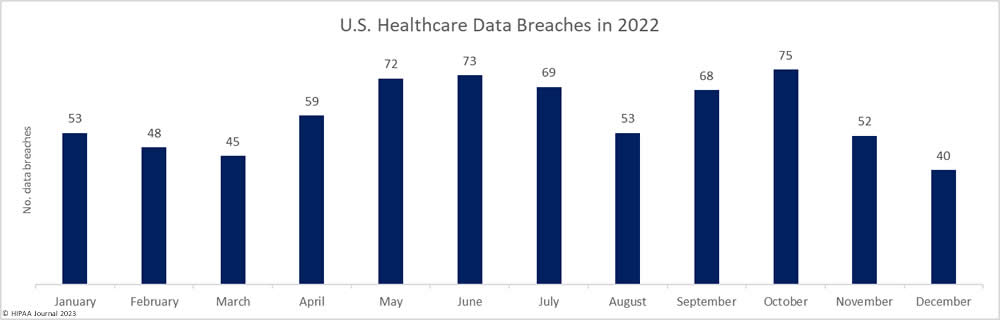
December 2022 Healthcare Data Breach Report

Prescription Drug Reporting Deadline Approaching

RxDC Reporting: What Plan Sponsors Need to Know - Word on Benefits

Billboard's 2022 Power List Revealed – Billboard

FDA National Drug Code: Proposed Format Changes & Industry Impact

Prescription Drug Reporting Deadline Approaching

This Year's Largest Healthcare Data Breaches

Prescription Drug Reporting Due by December 27, 2022

Data Protection Day – Key developments and trends for 2023 - Connect On Tech

Options for Reducing the Deficit, 2023 to 2032--Volume II: Smaller Reductions